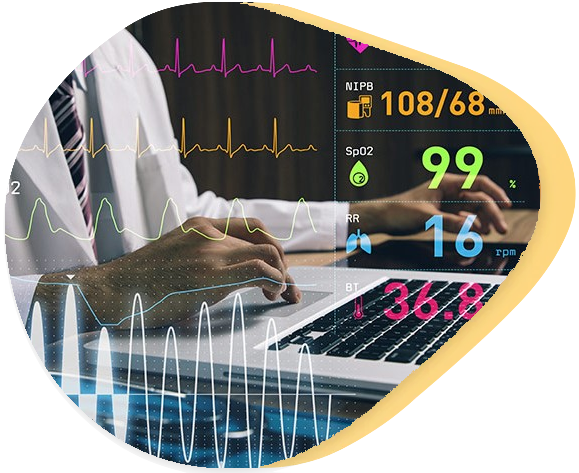
We have developed medical software components in various technologies ranging from PLCs (Siemens, Beckhoff), embedded (real-time applications) to desktop and web applications (JAVA, C#, JavaScript/Web). We are happy to advise you on the most appropriate architecture for your medical device.
Our team specializes in developing software for medical devices, ensuring compliance with industry standards and regulations. We leverage modern development practices to deliver high-quality solutions.
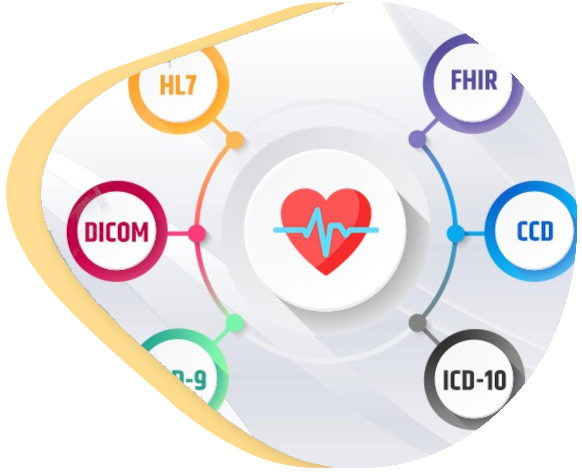

Medical device certification requires a safety-first approach. This means that at every step of the development lifecycle safety risks need to be examined and mitigated. This can sound like a never-ending process. We can help you do it effectively and safely, avoiding losing time-to-market due to missing documentation, evidence or other formalities.
... or we adapt to your specific needs.

We will analyze your use case, prepare a proposal and discuss it with you. It will include specifications of the work to be done, it will describe our approach, proposed technologies and architecture design, plus time and effort estimates.
We will implement the agreed-upon solution, following best practices and industry standards. Our development process is iterative, allowing for regular feedback and adjustments. And we are happy to work in collaboration with your team, use your tools and follow your processes, and spend time on-site if needed.


We will ensure a smooth launch of the product or a feature, providing support and monitoring to address any issues that may arise. Our goal is to help you achieve a successful deployment and have many happy users.
Ready to discuss your next project? Contact us and we'll help you achieve your goals.
We use cookies to enhance your browsing experience, analyze site traffic, and personalize content. By clicking "Accept", you consent to our use of cookies.